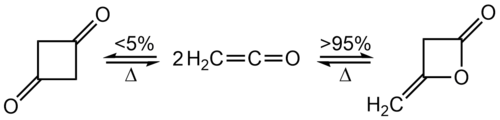Ketene

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule).[1] The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.[2]
Although they are highly useful, most ketenes are unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced.
History
[edit]Ketenes were first studied as a class by Hermann Staudinger before 1905.[3]
Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of -chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group).[4]
Properties
[edit]Ketenes are highly electrophilic at the carbon atom bonded with the heteroatom, due to its sp character. Ketenes can be formed with different heteroatoms bonded to the sp carbon atom, such as O, S or Se, respectively called ketenes, thioketenes and selenoketenes.
Ethenone, the simplest ketene, has different experimental lengths for each of its double bonds; the C=O bond is 1.160 Å and the C=C bond is 1.314 Å. The angle between the two H atoms is 121.5°, similar to the theoretically ideal angle in alkenes between sp2 carbon atoms and H substituents.[5]
Ketenes are unstable and cannot be stored. Absent nucleophiles with which to react, they dimerise (see § Reactions).
Synthesis
[edit]Ethenone is produced on a commercial scale by thermal dehydration of acetic acid. Substituted ketenes can be prepared from acyl chlorides by an elimination reaction in which HCl is lost:
In this reaction, a base, usually triethylamine, removes the acidic proton alpha to the carbonyl group, inducing the formation of the carbon-carbon double bond and the loss of a chloride ion:
Ketenes can also be formed from α-diazoketones by the Wolff rearrangement, and from vinylene carbonate by phosphorus(V) sulfide and irradiation.[6]
Another way to generate ketenes is through flash vacuum thermolysis (FVT) with 2-pyridylamines. Plüg and Wentrup developed a method in 1997 that improved on FVT reactions to produce ketenes with a stable FVT that is moisture insensitive, using mild conditions (480 °C). The N-pyridylamines are prepared via a condensation with R-malonates with N-amino(pyridene) and DCC as the solvent.[7]
A more robust method for preparing ketenes is the carbonylation of metal-carbenes, and in situ reaction of the thus produced highly reactive ketenes with suitable reagents such as imines, amines, or alcohols.[8] This method is an efficient one‐pot tandem protocol of the carbonylation of α‐diazocarbonyl compounds and a variety of N‐tosylhydrazones catalysed by Co(II)–porphyrin metalloradicals leading to the formation of ketenes, which subsequently react with a variety of nucleophiles and imines to form esters, amides and β‐lactams. This system has a broad substrate scope and can be applied to various combinations of carbene precursors, nucleophiles and imines.[9]
Ethenone can be produced through pyrolysis of acetone vapours over a hot filament in an apparatus that was eventually developed into the "ketene lamp" or "Hurd lamp" (named for Charles D. Hurd).[10]
Reactions
[edit]Due to their cumulated double bonds, ketenes are very reactive.[11] The free energy released in their saturation can power the formation of relatively strained rings.
Acylation
[edit]Ketenes are strong acylating agents. They react with carboxylic acids to form carboxylic acid anhydrides...
...with alcohols to form carboxylic acid esters...
...with amines to give amides...
...with water to give carboxylic acids...
...and with enolisable carbonyl compounds to give enol esters. For example, ethenone reacts with acetone to form a propen-2-yl acetate:[1]
Cycloadditions
[edit]As first observed in 1908,[12] ketenes react with virtually any electron-rich[13] π bond to form 4-membered rings.[1] For example, in the Staudinger synthesis,[14][15] a ketene attacks an imine to form a β-lactam:
Ketenes also cyclize onto enolic and enaminic alkenes, carbodiimides, and electron-rich alkynes (the latter forming cyclobutenones). cis Alkenes react more easily than trans alkenes.[16] Electron-withdrawing substituents on the ketene accelerate the reaction,[13] but disubstituted ketenes react slowly due to steric hindrance.[17]
Ketenes attack ketones and aldehydes to give β-lactones, but only under Lewis acid catalysis or when the carbonyl is electron-impoverished:[18]
Dienes generally react as two separate alkenes, and fulvenes typically react in the ring, leaving the exocyclic double bond intact:[19]
Stereochemistry
[edit][2+2] cycloadditions proceed by a concerted, thermal mechanism, which requires suprafacial- antarafacial alignment. Ketenes, unlike most alkenes, can align antarafacially with respect to other alkenes.[20] The unique transition state geometry has the interesting consequence that the bulkier substituent on the ketene will tend to end up on the more sterically hindered face of the cyclobutanone ring. In the transition state for cyclization, the small substituent points toward the alkene.
Ketenes place the larger substituent in the endo position when attacking cyclic alkenes.[21]
The use of chiral amine catalysts has allowed access to cycloaddition products in high enantiomeric excess.[22]
Higher-length cycloadditions
[edit]In rarer cases, ketenes may undergo [3+2], and [4+2] cycloadditions.[23]
[3+2] Cycloadditions may take place with 1,3-dipoles. This process appears to be concerted, but either ketenic double-bond can react.[24]
Michael acceptors often react in a [4+2] fashion:[25]
Conjugated ketenes may act as 4π partners in [4+2] cycloadditions as well.[26] Examples in which a vinylketene serves as the 4π partner are rare, but occur with some ketene-conjugated heterodienes:[27]
Dimerization
[edit]Ketenes autodimerize to give various products. The parent reacts acylates itself to form diketene, a β-lactone, whereas disubstituted ketenes undergo [2+2] cycloaddition to a substituted cyclobutadione:[28]
Monosubstituted ketenes can afford either the ester or diketone dimer.
Although many polar solvents and catalysts accelerate many reactions using ketene, such reactions are normally performed in nonpolar media to prevent dimerization.
Applications
[edit]Dimerization of stearic ketene affords alkyl ketene dimers, widely used in the paper industry.[1] AKD's react with the hydroxyl groups on the cellulose via esterification reaction.
Likewise, diols (HO−R−OH) and bis-ketenes (O=C=CH−R'−CH=C=O) react to yield polyesters with a repeat unit of (−O−R−O−CO−R'−CO).
The Staudinger synthesis is used to synthesize β-lactam antibiotics.[1]
Ethyl acetoacetate, an organic synthesis feedstock, is prepared industrially from diketene in ethanol.[citation needed]
See also
[edit]References
[edit]- ^ a b c d e Miller R, Abaecherli C, Said A, Jackson B (2001). "Ketenes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_063. ISBN 978-3527306732.
- ^ Saul Patai, ed. (1980). Ketenes, Allenes and Related Compounds: Part 1, Volume 1. PATAI'S Chemistry of Functional Groups. John Wiley & Sons. doi:10.1002/9780470771600. ISBN 9780470771600.Saul Patai, ed. (1980). Ketenes, Allenes and Related Compounds: Part 2, Volume 2. PATAI'S Chemistry of Functional Groups. John Wiley & Sons. doi:10.1002/9780470771617. ISBN 9780471276708.
- ^ Staudinger H (1905). "Ketene, eine neue Körperklasse" [Ketenes, a new class of substances]. Berichte der Deutschen Chemischen Gesellschaft. 38 (2): 1735–1739. doi:10.1002/cber.19050380283.
- ^ Thomas T. Tidwell, The first century of Ketenes (1905-2005): the birth of a family of reactive intermediates, Angewandte Chemie, Int. Edition, Band 44, 2005, S. 5778–5785
- ^ Ma NL, Wong M (2000). "A Theoretical Study of the Properties and Reactivities of Ketene, Thioketene, and Selenoketene". European Journal of Organic Chemistry. 2000 (8): 1411_1421. doi:10.1002/(SICI)1099-0690(200004)2000:8<1411::AID-EJOC1411>3.0.CO;2-N.
- ^ Handbook of Reagents for Organic Syntheses, Sulfur-Containing Reagents, ed. L.A. Paquette, Wiley-VCH, 2010, ISBN 978-0-470-74872-5, p. 535.
- ^ Carsten Plüg ,Hussein Kanaani and Curt Wentrup (12 February 2015). "Ketenes from N-(2-Pyridyl)amides". Australian Journal of Chemistry. 68 (4): 687. doi:10.1071/CH14714.
- ^ Paul ND, Chirila A, Lu H, Zhang XP, de Bruin B (September 2013). "Carbene radicals in cobalt(II)-porphyrin-catalysed carbene carbonylation reactions; a catalytic approach to ketenes". Chemistry: A European Journal. 19 (39): 12953–8. doi:10.1002/chem.201301731. PMC 4351769. PMID 24038393.
- ^ Chirila A, van Vliet KM, Paul ND, de Bruin B (2018). "[Co(MeTAA)] Metalloradical Catalytic Route to Ketenes via Carbonylation of Carbene Radicals" (PDF). European Journal of Inorganic Chemistry. 2018 (20–21): 2251–2258. doi:10.1002/ejic.201800101. ISSN 1099-0682.
- ^ Tidwell, Thomas T. (2005-09-12). "The First Century of Ketenes (1905–2005): The Birth of a Versatile Family of Reactive Intermediates". Angewandte Chemie International Edition. 44 (36): 5778–5785. doi:10.1002/anie.200500098. ISSN 1433-7851.
- ^ Siegfried Hauptmann (1985), Organische Chemie: mit 65 Tabellen (in German), Leipzig: Deutscher Verlag für Grundstoffindustrie, pp. 410–412, ISBN 3871449024
- ^ Frances Chick and Norman Thomas Mortimer Wilsmore (1908) "Acetylketen: a polymeride of keten," Journal of the Chemical Society, Transactions, 93 : 946-950.
- ^ a b Isaacs, N. S.; Stanbury, P. F. J. Chem. Soc., Chem. Commun. 1970, 1061.
- ^ Jie Jack Li (2006), Name reactions. A collection of detailed reaction mechanisms (in German) (3 ed.), Berlin: Springer-Verlag, pp. 561-562, doi:10.1007/3-540-30031-7, ISBN 9783540300304
- ^ Hermann Staudinger (1907), "Zur Kenntnis der Ketene. Diphenylketen", Justus Liebigs Annalen der Chemie (in German), vol. 356, no. 1–2, John Wiley & Sons, Inc., pp. 51–123, doi:10.1002/jlac.19073560106
- ^ Rey, M.; Roberts, S.; Dieffenbacher, A.; Dreiding, A. S. Helv. Chim. Acta 1970, 53, 417.
- ^ Huisgen, R.; Mayr, H. Tetrahedron Lett. 1975, 2965.
- ^ Metzger, C.; Borrmann, D.; Wegler, R. Chem. Ber. 1967, 100, 1817.
- ^ Stadler, H.; Rey, M.; Dreiding, A. S. Helv. Chim. Acta 1984, 67, 1854.
- ^ Moore, H. W.; Wilbur, D. S. J. Org. Chem. 1980, 45, 4483.
- ^ England, D. C.; Krespan, C. G. J. Org. Chem. 1970, 35, 3300.
- ^ Wynberg, H.; Staring, E. J. J. Am. Chem. Soc. 1982, 104, 166.
- ^ Hyatt, J. A.; Reynolds, P. W. Org. React. 1994, 45, 159. doi:10.1002/0471264180.or045.02
- ^ Texier, F.; Carrié, R.; Jaz, J. J. Chem. Soc., Chem. Commun. 1972, 199.
- ^ Mosti, L.; Menozzi, G.; Bignardi, G.; Schenone, P. Il Farmaco (Ed. Sci.) 1977, 32, 794 [C.A. 1978, 88, 62262n].
- ^ Staudinger, H. Die Ketene, Verlag von Ferdinand Enke, Stuttgart, 1912.
- ^ Jäger, G.; Wenzelburger, J. Justus Liebigs Ann. Chem. 1976, 1689.
- ^ Tenud, L.; Weilenmann, M.; Dallwigk, E. Helv. Chim. Acta 1977, 60, 975.
External links
[edit] Media related to Ketenes at Wikimedia Commons
Media related to Ketenes at Wikimedia Commons