Thorium triiodide
Appearance
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| I3Th | |
| Molar mass | 612.7511 g·mol−1 |
| Appearance | crystals |
| reacts with water | |
| Related compounds | |
Related compounds
|
Americium triiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thorium triiodide is a binary inorganic compound of thorium metal and iodine with the chemical formula ThI3.[1][2][3]
Synthesis
[edit]Th metal is heated with ThI4 in a vacuum at 800 °C.[4]
- Th + 3ThI4 → 4ThI3
Physical properties
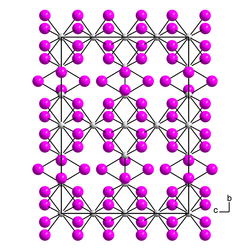
[edit]Thorium triiodide is a black, violet-tinged, usually poorly crystallized mass. Formed crystals exhibit strong dichroism from violet to olive green under the microscope and are birefringent.[5] Above 550 °C, thorium(III) iodide decomposes to thorium(IV) iodide and thorium(II) iodide. β-thorium(III) iodide has an orthorhombic crystal structure with the space group Cccm (space group no. 66).[6] The α-form has a pseudoorthorhombic crystal structure.
Chemical properties
[edit]ThI3 reacts with water.[4]
References
[edit]- ^ Yaws, Carl L. (6 January 2015). The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics. Gulf Professional Publishing. p. 802. ISBN 978-0-12-801146-1. Retrieved 3 April 2024.
- ^ Brown, David; Canterford, J. H.; Colton, Ray (1968). Halides of the Transition Elements: Halides of the lanthanides and actinides, by D. Brown. Wiley. p. 229. ISBN 978-0-470-10840-6. Retrieved 3 April 2024.
- ^ Seaborg, Glenn T. (20 May 1994). Modern Alchemy: Selected Papers Of Glenn T Seaborg. World Scientific. p. 21. ISBN 978-981-4502-99-3. Retrieved 3 April 2024.
- ^ a b David, Lore Rose (1953). Thorium: A Bibliography of Unclassified Literature. Technical Information Service. p. 18. Retrieved 3 April 2024.
- ^ Brauer, Georg (1975). Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume 1. Stuttgart: Ferdinand Enke. p. 1142. ISBN 978-3-432-02328-1.
{{cite book}}:|access-date=requires|url=(help) - ^ Morss, Lester R.; Edelstein, Norman M.; Fuger, J. The Chemistry of the Actinide and Transactinide Elements. Springer. p. 78. ISBN 978-94-007-0211-0.
{{cite book}}:|access-date=requires|url=(help)
